[026] Interaction of myosin and actin (GB#115B02) | 基礎医学教育研究会(KIKKEN)Lab

●Is there no real swing movement?
Muscle contraction occurs by shortening the bundle of protein fibers in muscle cells. Shortening of this bundle occurs not by shrinking of protein fiber molecules, but by movement (sliding) of the positional relationship between two types of protein fibers (filaments) lying next to each other, myosin and actin. This idea is called “sliding theory” of muscle contraction, 60 years passed since published by British researchers in 1954. Right now, there are (probably) no researchers who doubt the sliding theory themselves. The problem is how to get the power of that sliding movement. In almost all textbooks, myosin head is swinging lever arm model, which swings the actin by obtaining the energy of adenosine triphosphate (ATP). However, it seems that there are still many things we do not understand.
—
Many researchers in the world are continuing their research with a policy to elucidate the mechanism precisely on the premise that the myosin head is swinging. However, according to the breakthrough experiment of Professor Toshio Yanagida of Osaka University, it seems that the sliding of the filament will occur without actually swinging. So, someday the explanation of the textbook may be turned over at all. Thinking about it, it is cheerful to explain the head swing here, but there are also circumstances in which it will not be rewritten in the next few years, so let’s talk with the current textbook.
Contents
● Myosin’s head part is particularly important
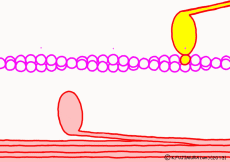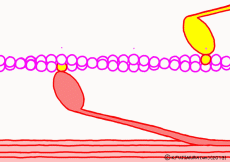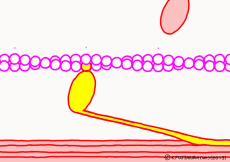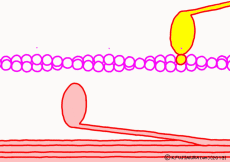
There are two types of muscle filaments in muscle cells: thick filaments in which proteins called myosin have been polymerized and thin filaments in which proteins called actin are polymerized. Polymerization is a way for molecules to fall apart to make a large agglomeration by making regular aggregates. One myosin consists of the head and the part of the long axis that follows, and the thick filaments are a bundle of parts of the shaft so that the head of each myosin faces outward. A thin filament made mainly of actin is located at the distance of the myosin head that can stick and leave. The state in which the myosin head is bound to actin is regarded as a bridge between a thick filament and a thin filament and is called crossbridge.
In fact, in addition to this, a protein that interferes with the reaction between myosin and actin while muscle contraction is not occurring constitutes the whole thin filament surrounding actin. When muscle contraction occurs, proteins interfering with troponin and tropomyosin are deformed by the action of calcium ions (Ca2+) that fly only when contracting. As a result, the reaction between myosin and actin proceeds. However, this time we omit the control mechanism of contraction and focus only on contraction mode.
● ATP separates myosin and actin
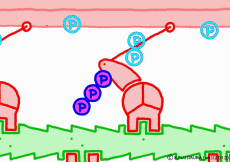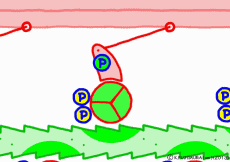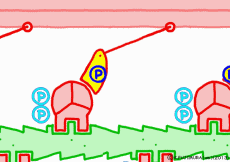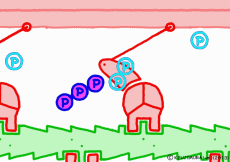
Well, the fact that ATP is the direct source of energy for muscle contraction is fundamental in the basic physiology. ATP binds to the myosin head. Myosin itself has the function of ATP degrading enzyme, decomposing ATP by binding with actin, taking out energy, and using that energy to draw actin. This is an overview of the mechanism of contraction, but if you hear that ATP is an energy source, you will have an image that myosin binds actin by binding ATP and releases actin when using energy. But, this is a primitive pitfall.
Actually, when myosin exhaust energy of the ATP, myosin remains strongly bound with actin (the strongly bound state). As ATP binds to myosin bound to actin, myosin releases actin first, as the structure of the molecule changes. It seems that it changes to a state called “weakly bound state” rather than completely releasing even if it says to release. Therefore, ATP is decomposed into adenosine diphosphate (ADP) and phosphoric acid, and energy goes out. This energy binds the phosphoric acid separated from ADP to myosin. It also serves as a force to advance the myosin head along the actin filament at the same time.
● Where is the power of myosin’s advancement?
At weak binding the myosin head moves the position “forward” along the actin filament. At this time, the original swinging theory seems to explain that the angle of the moving axle (lever arm) portion of the base of the myosin head changes and that the head is swung forward. However, if this is the case, the lever arm can not move the myosin head unless it is fixed to the main axis of myosin. Besides, in the state of weakly binding with actin, trying to move forward by swinging forward is hard to understand once again. It seems better to think that the force to advance the myosin head occurs not in the lever arm but in the part of the interaction with the actin molecule.
Indeed, in a report by RIKEN, it is explained that a force of movement occurs between the proximal myosin head and the amino acid sequence of actin, changing the angle of the molecular structure of the myosin head. Therefore, in this animation, the tip of the myosin head is drawn in the image which reacts with actin and advances. What is spinning is ‘expression’ to emphasize ‘actively advancing’, in fact the molecules do not rotate. It seems that it is going through unnoticedly making irregular vibrations in small increments. It looks like a recent ultrasonic motor.
● Actin filament has orientation
By the way, does myosin head know which one of actin fibers is ahead? The answer is simple. For each actin molecule making up fiber, it says this before and this one behind. Seriously, the actin molecule has directionality and it is connected in tandem in the same direction, so you can also see the direction of the fiber. In this case, for myosin, the front of the actin fiber is the side on which the actin fiber is fixed to the Z membrane, which is the boundary of the sarcomere. The direction of actin is expressed as + end and – end, and myosin advances from – end toward + end.
● The last row pulls the filament
When the myosin head advances to the appropriate position on the actin filament with the energy of ATP, the relationship between myosin head and actin changes to “strong binding state”. The energy generated by the first ATP decomposition has just been exhausted. However, phosphoric acid still has high energy binding to myosin head. Therefore, as the shape of the myosin itself changes due to the movement of the myosin head, the bound phosphoric acid is now separated from the myosin, and the lever arms are made to the original angle by the energy generated at that time return. At that time the entire filament is drawn a little, and the sarcomere is shortened by one stroke. As phosphoric acid goes apart and the lever arm returns to its original shape, ADP that remained in the head of myosin on a signal as it came away. This is one cycle of filament sliding due to the interaction between myosin and actin. This cycle repeats as ADP goes away as new ATP joins the myosin head in exchange, entering the next cycle and contraction of the muscle.
There seems to be various opinions about how much myosin pulls actin with one molecule of ATP, but since it depends on the resistance (load) applied to the muscle fiber originally, there is nothing to worry about at the vocational school level (I think).
● Even if you lose power, ATP is overflowing
If you are healthy, you will not run out of ATP on your way while you are contracting your muscles. Of course, using muscles gradually becomes tired and the force does not come out. But, “vitality” has disappeared long before ATP disappears, your muscle cells will go from the contractile mode to the relaxation mode, as the nerve command ceases.Among the muscle cells, ATP is prepared as needed at any time. If it is not enough, cell activity will be mobilized and replenished. So even when your muscle filaments are relaxed, there are lots of ATP around myosin. It is not the presence or absence of ATP that determines muscle contraction and relaxation, but without the presence of calcium ions (Ca2+), this is taken up on another occasion.
If the next ATP is gone when one cycle of myosin head movement is over, myosin and actin remain strongly bound. When muscle cells died, supplementation of ATP ceases, so the muscle filaments in a muscle cell stiff and stiff all at once. It is written in most textbooks that this is a mechanism of “stiff rigidity”. Especially, ATP is exhausted as soon as the muscle contracting strongly at the moment of death, it seems to get harder soon.
◎ Description for the animation: Note: Although we are expressing myosin with a lever arm and a wheel in this animation, in fact it is a mass of one continuous molecule. The figure of ATP is represented with only three phosphate groups, omitting adenosine. The portion of myosin to which ATP binds is actually on the reverse side (the side opposite to the direction of advancement), but it was difficult to express graphically, so it is in front of myosin. In the figure, actin is on the plus side in the left direction.
○ People who are interested in detailed explanation should look into it.
⇒ Elucidation of energy conversion mechanism of muscle contraction. “Mechanism of myosin’s force generation caused by actin binding has been completely elucidated at the molecular level.” (July 23, 2007, Japan Science and Technology Agency (JST), RIKEN)
⇒How do proteins move the body: (broken link: 20170701)
⇒ Structure of myosin:
⇒ Watch muscle movements with one molecule – cross the machine? Myosin · motor: Yanagida Toshio:
⇒ See the wonder of life from biological motors:
⇒ Is Myosin’s “Tail swing theory” correct? :
⇒ Lever arm movement and power stroke of myosin motor protein
○ Related articles
◆[019] アデノシン三リン酸(ATP) adenosine triphosphate ![]()
◆[009] 筋収縮の伸縮幅 the range of muscular contraction ![]()
◆[035] 骨格筋収縮の張力 tension of the skeletal muscle contraction ![]()
◆[033] 平滑筋の収縮 smooth muscle contraction ![]()
◆[041] 心筋線維 myocardial fiber ![]()
◆[021] Action potential ![]()
○ Referenced books
・プロッパー細胞生物学,化学同人
・Essential細胞生物学〈DVD付〉原書第3版,南江堂
・細胞の分子生物学, ニュートンプレス; 第5版 (2010/01)
・肉単―ギリシャ語・ラテン語 (語源から覚える解剖学英単語集 (筋肉編))
・カラー図解 人体の正常構造と機能 全10巻縮刷版,坂井 建雄,日本医事新報社
・人体機能生理学,杉 晴夫,南江堂
・トートラ人体解剖生理学 原書8版,丸善
・イラスト解剖学,松村 讓兒,中外医学社
・柔道整復学校協会編「生理学」,南江堂
・東洋療法学校協会編「生理学」,医歯薬出版株式会社
rev.20150721,rev.20170505.
KISO-IGAKU-KYOIKU-KENKYUKAI(KIKKEN)