[006] Osmotic pressure (GB#101F01) | 基礎医学教育研究会(KIKKEN)Lab

● Cell is a bag that draws water
It is the water that has the largest amount anyway with the constituents of the living body. A liquid in which something is dissolved in water is called an aqueous solution. A substance dissolved in water is called a solute. The properties that will be described in the following are fundamentally no different for any solution. On the other hand, the original liquid dissolving the solute is called a solvent. In the case of a living thing, as the solvent is determined to be water, let’s go with water as it is.
—
Contents
- 1 ● Cell is a bag floating in salt water
- 2 ● Power to withdraw water, osmotic pressure
- 3 ● Osmotic pressure is determined by the “number” of particles
- 4 ● Pickles, salted foods, osmotic pressure
- 5 ● Ikebana, watering of the plants, osmotic pressure
- 6 ● Osmotic pressure working through the wall made of cells
- 7 ○ Referenced sites
- 8 ○ Related articles
- 9 ○ Referenced books
● Cell is a bag floating in salt water
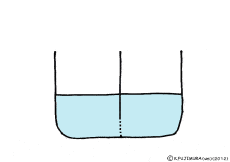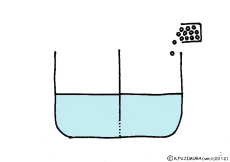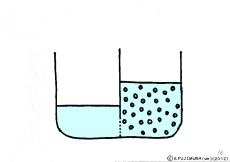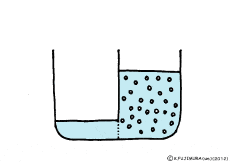
When the solute is fully dissolved in water, a thick aqueous solution is formed. If it is not dissolved too much, it becomes a thin aqueous solution. Around the cell, roughly speaking, it is a thin salt water (in terms of human taste). On the other hand, cells are bags wrapped in special membranes called cell membranes, in which various substances are dissolved.
If the thick aqueous solution and the thin aqueous solution are blocked by each other, for example, by the wall of the glass plate, nothing happens. It is the same as being contained in two separate glass beakers.
When this glass plate disappears, the two aqueous solutions mix together, and solutes that are dissolved from the thick solution to the thin solution will spread. It is because the natural forces in the direction of the same concentration are working between the two solutions. When it is a glass plate, each other’s power does not work because it is completely separated.
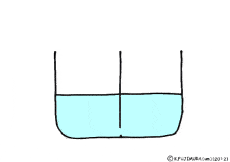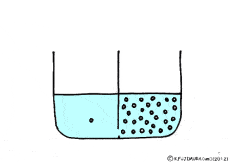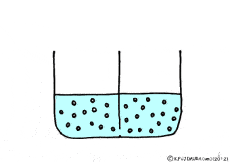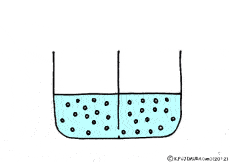
Where is the existence of the blocking walls kept intact, if the properties of the walls change? What it will be.
If the wall could not let the solute pass but molecules of water could pass through it.
Is there such a wall? The membrane of the cell making the organism is like that.
Though water molecules let through, solutes do not pass, such a special membrane is called semipermeable membrane.
Since semipermeable membranes are not completely obstructing, selected substances pass through and force acts in the direction that the two solutions have the same concentration.
Let’s consider a case where a thick solution and a thin solution are in contact with each other through a semipermeable membrane.
If these solutions are going to be at the same concentration, the high concentration solution will change to a lower and the lower concentration solution will try to get a higher. A thick aqueous solution adds water and it becomes a thin solution. A thin solution will draw a thick solution if it sucks out only water. So, even if the solute can not move, as the water moves from a thin solution to a thick solution, it will approach the target state. Indeed, in this way water movement from the thin solution to the thick solution naturally occurs through the semipermeable membrane. This is a phenomenon called osmosis.
(Incidentally, the membrane through which everything passes is called the total permeable membrane.)
● Power to withdraw water, osmotic pressure
Osmosis becomes the power to move water. This force uses the word osmotic pressure. This word often appears in physiology. So it is an important word, but it is difficult to explain this osmotic pressure. Originally, osmotic pressure is said to be the pressure required to stop movement of water. Water penetrates into the aqueous solution from fresh water when the fresh water that is not melted and the aqueous solution of something are obstructed by the semipermeable membrane. As osmosis occurs, the amount of aqueous solution will increase by that amount. It seems that the pressure applied in the reverse direction so that this does not increase is the value of the osmotic pressure. But even if such a thing is told, it is confusing and can not be helped.
I am simply teaching that osmotic pressure is the power to draw water. Since the osmotic pressure of the solution is a value for fresh water, it is a fixed value for each solution. The osmotic pressure of the thick solution is high and the osmotic pressure of the thin solution is low. Osmotic pressure is treated almost like “concentration”. What happens when a solution with high concentration and a solution with low concentration come in contact via a semipermeable membrane? When a solution with a high osmotic pressure and a solution with a low osmotic pressure are in contact with each other through a semipermeable membrane, water is sucked into each other, but one with a higher osmotic pressure, that is, one with a higher concentration sucks in more water. Even if the expression is different, the phenomenon of the result is the same.
Speaking of “pressure”, there is generally an image of pressing force, and there may be actual technical problems, perhaps as a pressure to stop penetration. However, it is easier to explain that the aqueous solution draws water.
● Osmotic pressure is determined by the “number” of particles
Because osmosis is not the property of dissolved solute but the nature of particle and semipermeable membrane, osmotic pressure is the same if particle density is the same even if various solutes are mixed. One ion of sodium, one molecule of glucose, and one molecule of protein are completely different in size and property, but if it is a substance that can not pass through a semipermeable membrane, it is treated as one particle.
Very different solutes are dissolved at different ratios inside and outside the cell membrane. The osmotic pressure has been almost the same in each solution, all together. Although it is almost the same, slightly, the osmotic pressure inside is raised, so the cell sucks water from the surroundings slightly and keeps its shape with a slightly bulging state. (← This expression may be slightly strange. In addition to the osmotic pressure, various transport systems work on the cell membrane, and in the stable state the pressure inside and outside of the membrane on the cell membrane as a whole is just balanced and the cells do not swell or shrink. Plant cells with a firm cell wall on the outside utilize the expansive force due to osmotic pressure to keep the whole plant stiff.)
● Pickles, salted foods, osmotic pressure
Sodium salt ion and miso amino acid are basically substances that can not pass through the cell membrane. Therefore, salted around the cell, when pickled in miso, water is absorbed from the cell with a strong force. Pickling is wrinkle because water is drained. Amino acids and nutrients in the cells remain.
● Ikebana, watering of the plants, osmotic pressure
When cells are placed in a very thin aqueous solution, the difference in osmotic pressure increases, so the cells suck more water. When a scintillated cut flower is soaked in water, the wilted cell absorbs water and swells and fills up. If the degree of wilting is severe, the cell membrane is broken, so it will not be enough anymore.
Since each cell of a plant is contained in a box that does not grow and shrink, even if the cell is filled with water, it can not expand any more. On the other hand, animal cells tend to expand beyond their original size if the force to inhale water is too great. However, if the cell membrane is pulled too much, it is a substitute that can be easily torn rather than stretching. If the cells are in close contact with each other, they keep pushing each other still, but if they are cells that are separately active, they will burst too much and rupture.
* A phenomenon that erythroid cells rupture by touching a solution with low osmotic pressure is called “hemolysis”. As the cell breaks and the red hemoglobin in it scatters and disappears, it seems that the cell dissolves and disappears.
● Osmotic pressure working through the wall made of cells
Osmotic pressure usually refers to the fundamental important force working against each cell. However, osmotic pressure through the wall as a tissue created by gathering cells is more frequent in physiology textbooks. It’s a capillary wall.
The capillary wall clearance is much larger than the cell membrane gap. So water of plasma comes in and out through the wall of the tube. Ions also enter and leave comfortably. So there is no difference in ion concentration between inside and outside the capillary. On the other hand, protein in the blood basically does not go out much. Therefore, protein concentration is high in capillaries and low outside. Therefore, pressure called plasma colloid osmotic pressure works. Plasma colloid refers mainly to proteins dissolved in plasma. The power to inhale water from around the blood vessel due to the protein dissolved in the plasma, this is the plasma colloid osmotic pressure. In other words, water goes out from capillary vessels, but on the other hand it also works to inhale water by the action of plasma colloid osmotic pressure.
○ Referenced sites
⇒Those who want to know more about osmotic pressure
⇒If you want to know more about plasma colloid osmotic pressure
⇒When you want to know about osmotic pressure measurement method
⇒If you would like to know more about cell membranes
○ Related articles
◆[013] 細胞膜の脂質二重層 lipid bilayer of the cell membrane ![]()
◆[004] 陽イオンと陰イオン(1)引力と反発力,cation and anion, attraction and repulsion ![]()
◆[037] 膠質浸透圧 colloid osmotic pressure ![]()
◆[043] 糸球体のろ過 glomerular filtration ![]()
◆[024] Voltage-dependent sodium channel ![]()
◆[028] Resting membrane potential ![]()
◆[017] 糖質の吸収 absorption of carbohydratel ![]()
○ Referenced books
・カラー版 ボロン ブールペープ 「生理学」,西村書店 (2011)
・カラー図解 人体の正常構造と機能 全10巻縮刷版,坂井 建雄,日本医事新報社
・人体機能生理学,杉 晴夫,南江堂
・トートラ人体解剖生理学 原書8版,丸善
・イラスト解剖学,松村 讓兒,中外医学社
・柔道整復学校協会編「生理学」,南江堂
・東洋療法学校協会編「生理学」,医歯薬出版株式会社
rev.20170205,rev.20170502, rev.20180313.
KISO-IGAKU-KYOIKU-KENKYUKAI(KIKKEN)