[028] Resting membrane potential (GB#113B01) | 基礎医学教育研究会(KIKKEN)Lab

● Ions through the cell membrane determine membrane potential
A living cell has a negative voltage inside the cell membrane and a positive voltage on the outside. It is supposed to measure the voltage inside from the outside. This voltage is called membrane potential. Although the value of the membrane potential may change depending on the activity of the cell, the membrane potential of a stable state with no particular fluctuation is called resting membrane potential or resting potential. The resting membrane potential is roughly -50 mV to -100 mV, which is common to all living cells, and all cell activities are performed under this environment.
—
Contents
- 1 ● How difficult is membrane potential?
- 2 ● Ion components are different between cell membranes
- 3 ● If there is no leakage of ions there is no potential
- 4 ● Ion will leak and electric potential will be generated
- 5 ● Polarization and depolarization
- 6 ● Diffusing ions do not have the same concentration
- 7 ● Polarity easily reverses
- 8 ● There is energy to maintain membrane potential
- 9 ○ Related articles
- 10 ○ Referenced books
● How difficult is membrane potential?
 However, looking at the textbook of “cell biology”, there is only a slight touch to “mechanism of membrane potential”. On the other hand, if you look at textbooks such as “neurophysiology”, there is detailed description, but explanation is too correct (laugh), then, no one can understand almost. After all, it seems that many people have doubts while passing by unknown. However, it seems that it is not too difficult to think.
However, looking at the textbook of “cell biology”, there is only a slight touch to “mechanism of membrane potential”. On the other hand, if you look at textbooks such as “neurophysiology”, there is detailed description, but explanation is too correct (laugh), then, no one can understand almost. After all, it seems that many people have doubts while passing by unknown. However, it seems that it is not too difficult to think.
● Ion components are different between cell membranes
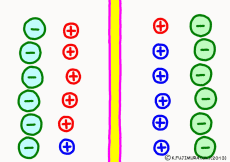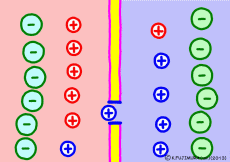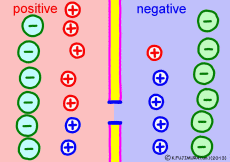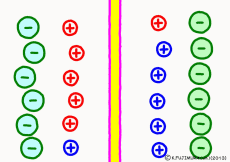
A large amount of ions are dissolved in the intracellular and the extracellular solution sandwiching the cell membrane. However, the contents of both solutions are not even. In the extracellular solution consisting of interstitial fluid, lymph fluid, blood, etc, the main ions are sodium ion (Na ion, Na+) and chloride ion (Cl– ). Even junior high school students know this well. However, the main cation (positive ion) of the cell is not sodium ion but potassium ion (K+). Conversely, outside of the cell, K+ is only about one-tenth of Na+. In other words, the concentration of Na+ and K+ across the cell membrane are significantly different, and are reversed completely. If the cell membrane allows ions to pass freely, the two ions will mix equally by diffusion. However, the lipid bilayer membrane making up the cell membrane can not pass ions at all as a basic property. Since it does not pass ions, even this uneven ion distribution does not diffuse and remains intact. However, there is actually no phenomenon called membrane potential if it really does not pass ions (???). What? Would not this be an explanation for the membrane potential? !
※ In animation, ions are arranged vertically because they make it easy to understand numbers, in fact the ions should move around freely.
● If there is no leakage of ions there is no potential
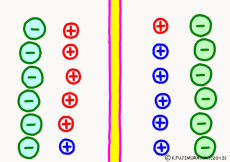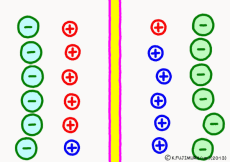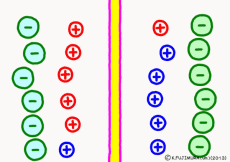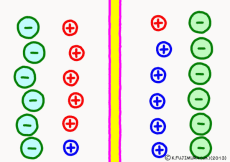
Originally, the aqueous solution is very severe for positive or negative electricity deviations. The type of ion is fine, but unless there are special circumstances, the aqueous solution is in a bad mood unless the positive and negative sums of the ions are the same. If it is the same number, it is deducted, but if it is not the same number, it means that the aqueous solution itself has positive or negative static electricity. Without particular reasons, the static electricity will not remain, as static electricity in the aqueous solution is activated immediately by a slight chance. The state that the aqueous solution as a whole does not have electricity is stable and correct.
Now a membrane that does not pass ion separates the two kinds of aqueous solution. As shown in the animation, each aqueous solution has 6 positively charged cations, and 6 anions ![]() that are balanced with them are dissolved. A total of 12 ions are dissolved, and the concentration of the whole ion is the same for both the right and the left solution. (Somewhat differences in the concentration of the whole ion do not significantly affect the mechanism of the potential generation, but we make it the same concentration for the sake of simplicity, in fact it is almost the same.)
that are balanced with them are dissolved. A total of 12 ions are dissolved, and the concentration of the whole ion is the same for both the right and the left solution. (Somewhat differences in the concentration of the whole ion do not significantly affect the mechanism of the potential generation, but we make it the same concentration for the sake of simplicity, in fact it is almost the same.)
However, there are two types of cations, here red is Na+ ion![]() , blue is K+ ion
, blue is K+ ion![]() In this state, when two solutions are separated by a membrane which is completely impermeable to ions, both solutions are the same as placed in separate glass bottles and do not act on each other. Even if the components of the cations are different, it is not related at all, it is the same as opening the terminals of the voltmeter, and the voltage between the two solutions can not be measured.
In this state, when two solutions are separated by a membrane which is completely impermeable to ions, both solutions are the same as placed in separate glass bottles and do not act on each other. Even if the components of the cations are different, it is not related at all, it is the same as opening the terminals of the voltmeter, and the voltage between the two solutions can not be measured.
● Ion will leak and electric potential will be generated
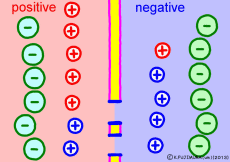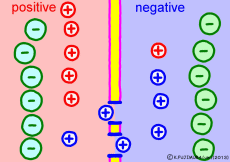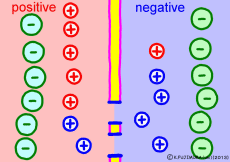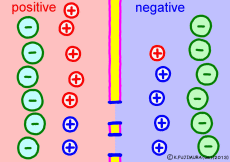
Cell membranes of living cells are different from just lipid bilayer membranes. There are many differences, but the important difference related to the generation of electric potential is that it has the property of passing only “specific ions”. This is due to the fact that proteins called ion channels that pass only specific ions are embedded in the lipid bilayer membrane. Every cell membrane has a “K+ ion channel” that passes only K+ ions as its basic item.
Numerous K+ ion channels are opening the cell membrane. As a result, the cell membrane can not pass other ions, but K+ ion ![]() Then the situation changes completely. The concentration of K+ ions in the membrane is very different. The more the blue K+ ion, the more intracellular. Because K+ ion is small outside the cell, intracellular K+ ion will try to diffuse outward as a natural flow. In fact, it flows out through ion channels (only a few but …). However, the K+ ion has positive electricity rather than just particles. Originally, if only ions with positive electricity come out from the solution that plus and minus should be balanced, negative electricity will remain in the remaining solution. On the other hand, plus electricity exceeds extracellular solution. So if the concentration of K+ ions is small outside the cell and there is a concentration bias in the cell, then the inside of the cell will become negative.
Then the situation changes completely. The concentration of K+ ions in the membrane is very different. The more the blue K+ ion, the more intracellular. Because K+ ion is small outside the cell, intracellular K+ ion will try to diffuse outward as a natural flow. In fact, it flows out through ion channels (only a few but …). However, the K+ ion has positive electricity rather than just particles. Originally, if only ions with positive electricity come out from the solution that plus and minus should be balanced, negative electricity will remain in the remaining solution. On the other hand, plus electricity exceeds extracellular solution. So if the concentration of K+ ions is small outside the cell and there is a concentration bias in the cell, then the inside of the cell will become negative.
● Polarization and depolarization
By the way, the state where positive and negative are separated like this is called polarization. If the cell membrane has the property of releasing the inner anion together with the K+ ion, it is needless to say that polarization does not occur since the movement of electricity is net zero.
The degree of polarization decreases for some reason is called “depolarization” (depolarization). On the other hand, the fact that the degree of polarization becomes large is called “hyperpolarization”.
You should be careful when you encounter the expression “membrane potential increases”. The higher the potential, the higher the same meaning if the degree of polarization is large. So it should represent a “hyperpolarized” state. On the other hand, the expression that the membrane potential is low or small means that the degree of polarization is small, that is, the state of “depolarization”. However, among some experts, people are dragged by the image above and below the graph of the membrane potential, and some people say that the potential becomes “high” when “depolarizing” is confused sometimes. When the story breaks, it is better to check depolarization or hyperpolarization. Of course, in the Kikken-lab, “the potential decreases” means “depolarization”.
● Diffusing ions do not have the same concentration
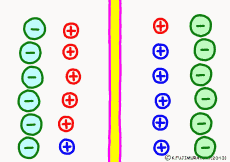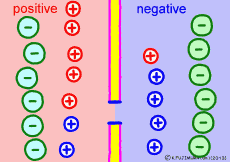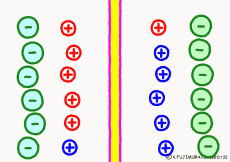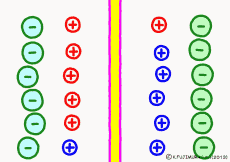
The driving force that makes the intracellular potential negative is the diffusing power of a large amount of K+ ions in the cell. This works to equalize the K+ ion concentration inside and outside the membrane. However, if we try to make the concentrations the same, the electrical balance will collapse inside and outside the membrane this time. K+ ions tend to flow out, the potential difference between the inside and the outside is unnecessarily increased, The ions will be pushed back.
So, K+ ions eventually settled naturally at the point where they got a balance of concentration and an electrical balance. Where to settle on the periphery is the difference in concentration of K+ ions inside and outside the membrane, on the formula (Nernst’s formula or Goldman-Hodgkin-Kats formula), the logarithm of the concentration ratio . If it says roughly, the larger the difference in concentration, the larger the potential difference, and the smaller the difference in concentration, the smaller the potential difference. If there is no difference in concentration, the potential difference is zero and the membrane potential disappears. If there is a concentration difference between the membrane that allows only K+ ions to pass and the concentration of K+ ions inside and outside the membrane, the resting membrane potential naturally occurs physically it’s a simple mechanism.
* For supplements, for Na+ ions that do not have paths here, the resting cell membranes are only walls where the other side is invisible. Na ion does not bother the difference of “concentration” with the other side.
With ordinary cells, there are about 30 times more extracellular K+ ions in the cell, and the resting membrane potential is about -50 mV to -90 mV in magnitude. As I wrote in the beginning, this is the magnitude of the intracellular potential measured with the extracellular potential as zero. Even if it is the smaller -50 mV, this is one twentieth of 1 V (= 1000 mV), so I wrote it at the action potential article, but this is quite a large voltage level for the ultrathin cell membrane.
● Polarity easily reverses
Ion components and concentrations inside and outside the cell are not changed so much as long as they are healthy. However, the ease of passing ions varies greatly depending on the activity of the cells. The ion channel has a lid that opens and closes, and the K+ ion channel is a stationary open channel. There are channels that allow other ions to pass through the cell membranes, but they are closed and not functioning.If there is a channel that allows only Na+ ions to pass (K+ ions can not pass) channel and it is only open, K+ ions, the polarized state is reversed, the extracellular portion of Na+ ions will be negative, the interior of the cell will be positive. The logic is the same. The diffusion power of Na+ ion makes plus inside the cell.
For the purpose of explanation, it is decided that the cell membrane does not pass K+ ions but only Na+ ions. Actually, in order to decide which direction the polarization will swing, it is not necessary to completely oblique either ion. It depends on which cell membrane is more likely to pass either ion. When the Na+ ion becomes easier to pass through than the ease of passage of the static K+ ion, the potential reverses at that moment.
The action potential of excitable cells such as nerve cells and muscle cells is generated by opening the Na+ ion channel more than 20 times the K+ channel simultaneously all at once. If the open Na+ ion channel closes all together, the cell membrane will return to the property of passing only K+ ions and at the same time the membrane potential will return to the resting state . The mechanism by which both resting membrane potential and action potential were based was the same.
● There is energy to maintain membrane potential
The resting membrane potential is presumed to be a large concentration difference between the membrane that allows only K+ ions and the K+ ion inside and outside the cell membrane. If it is an ideal cell membrane that permits only K+ ions at all, once the resting membrane potential is stabilized, the entrance and exit of ions is stabilized, so that the potential and the concentration difference are maintained as it is. However, the actual cell membrane has some leakage against other ions. Especially Na+ ions are about to flow into the cell if there is a pocket. At the action potential positively opens the gate for Na+ ions. By doing so, K+ ions are more likely to flow out as much as the Na+ ion of the same cation enters. So, as time passes, ions inside and outside the cell will mix up and the membrane potential will also decrease.
As long as the cell is alive, it must evacuate the Na+ ion in the cell and take K+ ion from the outside of the cell to keep this concentration distribution. This work is carried out by a device called a sodium-potassium ion pump, which is also in the cell membrane, apart from ion channels. This is a representative example of active transport that operates using energy of adenosine triphosphate (ATP). Without this, membrane potential can not be maintained for a long time. Therefore, some books have cited the existence of a sodium potassium ion pump as the “cause” of the resting membrane potential (although the real is only indirectly involved …⇒ Physically and chemically describing the cause of the resting membrane potential, due to the membrane permeability of the K+ ion and the concentration difference, biologically, it is due to the sodium ion pump, it may be).
The concentration distribution of intracellular and extracellular K+ and Na+ ions was not made without any effort.
※ Although it is a simple story, it has become long last (laugh). Thank you very much for keeping me till the end.
・About static electricity ⇒Miscellaneous science note: – Story of static electricity –
○ Related articles
◆[021] Action potential ![]()
◆[031] 興奮伝導 conduction of excitation ![]()
◆[024] Voltage-dependent sodium channel ![]()
◆[040] シナプス伝達 neural signal transmission ![]()
◆[036] Ligand-gated ion channel ![]()
◆[053] Acetylcholine receptors (GB#114B03) ![]()
◆[013] 細胞膜の脂質二重層 lipid bilayer of the cell membrane ![]()
◆[006] Osmotic pressure ![]()
◆[019] アデノシン3リン酸(ATP) adenosine triphosphate ![]()
◆[004] Cation and anion, attraction and repulsion ![]()
◆[017] 糖質の吸収 absorption of carbohydratel ![]()
○ Referenced books
・プロッパー細胞生物学,化学同人
・Essential細胞生物学〈DVD付〉原書第3版,南江堂
・細胞の分子生物学, ニュートンプレス; 第5版 (2010/01)
・肉単―ギリシャ語・ラテン語 (語源から覚える解剖学英単語集 (筋肉編))
・カラー図解 人体の正常構造と機能 全10巻縮刷版,坂井 建雄,日本医事新報社
・人体機能生理学,杉 晴夫,南江堂
・トートラ人体解剖生理学 原書8版,丸善
・イラスト解剖学,松村 讓兒,中外医学社
・柔道整復学校協会編「生理学」,南江堂
・東洋療法学校協会編「生理学」,医歯薬出版株式会社
rev.20140308, rev.20140705,
rev.20150503, rev.20160208, rev.20160711,rev.20170505, rev.20171222, rev.20180108.