[027] Peptide bond (GB#101D02) | 基礎医学教育研究会(KIKKEN)Lab
●Amino acids are all connected in the same way
A component of a protein is an amino acid, and a protein is a macromolecule formed by connecting amino acids in a chain. There are twenty kinds of amino acids that become parts in our body, and depending on the number, kind and order of the constituent amino acids, the shape and workability of the protein to be formed are almost decided. Each of the 20 kinds of amino acids has a different molecular shape, but the great thing is that all the amino acids can be linked in one and the same way.
—
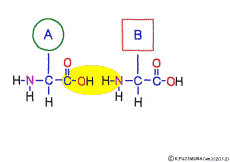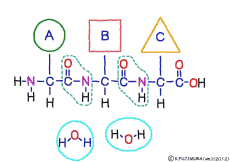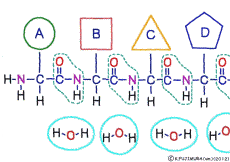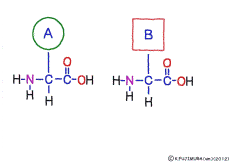
This linkage is one of dehydration condensation which water molecules form when connected, and is named peptid bond. Peptide is a large molecule formed by connecting amino acids.
Contents
- 1 ●Peptide and protein are the same?
- 2 ● Each amino acid has its own personality
- 3 ● Amino acids have the same coupler
- 4 ● Peptide has front and back
- 5 ● Use energy to link amino acids
- 6 ● Amino acids are chosen one by one and concatenated
- 7 There is a fixed beginning of polypeptide
- 8 ● Peptides are hydrolyzed to separate amino acids
- 9 ● Peptide decomposition is unexpectedly troublesome
- 10 ○ Referenced sites
- 11 ○ Related articles
- 12 ○ Referenced books
●Peptide and protein are the same?
Since the compound formed by linking amino acids is a peptide, the protein is also a peptide, but it seems that it is usually said that a molecule with a small number of amino acids is a peptide and a large molecule is a protein. Approximately 50 or more are likely proteins. However, in another book there are more than 20 things called proteins.It is also called a slightly larger peptide (oligopeptide), pretty big peptide (polypeptide). Although it seems to be a polypeptide like 10 or more, it is difficult to establish clear boundaries.
Additional note: According to the website of the Ministry of Education, Culture, Sports, Science and Technology, “Peptide with about 2 to 20 amino acids bound together is called oligopeptide (IUPAC & IUBMB-1983)”. (2018-04-27)
Besides, there are various peptide designations such as peptides of two amino acids (dipeptide, dipeptide), three amino acid peptides (tripeptide, tripeptide). However, there is nothing to do with the size and the weight of work. Peptides made with (healthy) cells are all strictly controlled in order and kind of amino acids, irrespective of size, it can be said that they have important work as it is. The term protein is said to mean that the polypeptide has been further functionalized.
(From now on, we will represent proteins as well as proteins of various sizes together as polypeptides.)
● Each amino acid has its own personality
There are two ways to divide roughly 20 kinds of amino acids which are parts of polypeptides. Amino acids that are familiar with water molecules (hydrophilic) and water molecules are poorly balanced (hydrophilic and hydrophobic) amino acids. Hydrophilic amino acids include basic amino acids and acidic amino acids.Basic amino acids generate hydroxide ions (OH–) and acidic amino acids generate hydrogen ions (H+). Therefore, amino acids themselves have positive and negative electricity respectively. There are also hydrophilic amino acids without electricity. In addition to its basic properties, each amino acid has its own characteristic and role, its characteristics and structure are disjoint. On the other hand, however, amino acids have an important structure that is common to all.
● Amino acids have the same coupler
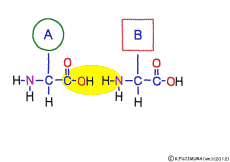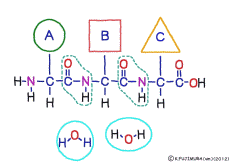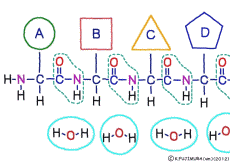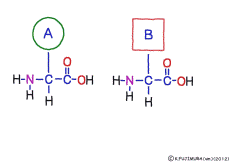
The characteristic of an amino acid is that it always has a fixed form of (NH2 -CH -COOH) for linking at one end of its structure. NH2 on the left side is an amino group, COOH on the right side is a carboxy group (it is said to be a carboxyl group in the past but it seems that it was changed by international standards beforehand) is there. Then, one side of the amino group is called the amino terminal (N-terminus, N-terminal end) and the side having the carboxy group is called the carboxy terminal (C-terminus, C-terminal end). When drawing a single amino acid, it is a promise to draw the N terminus on the left side and the C terminus on the right side. The N-terminus and the C-terminus are separated from each other at each amino acid, but the N-terminus is made to firmly bind to the C-terminus of another amino acid and the C-terminus to the N-terminus of another amino acid. The important point is that the bond itself is in the same format as – COOH vs. NH2 – both right and left. This results in a peptide bond of -CONH-.
In other words, there are various kinds of amino acids, but all can be connected by the same mechanism. Conversely speaking, it is possible to make several different polypeptides in the same connection way.
● Peptide has front and back
There are differences between the N-terminus and the C-terminus of peptide bonds such as the anterior side and the posterior side, so that the structure of a polypeptide connected in series also has anterior side and posterior side. The head of the peptide is N-terminal side. This depends on the mechanism when the polypeptide is made.When a polypeptide is made in a cell, it can only use a method of linking a new amino acid to the C-terminus of the original amino acid or peptide. In general, the introduction will be incredibly long to explain its mechanism. Separate that long preamble separately, let’s focus only on where to connect.
● Use energy to link amino acids
Not only peptide bonds but also substances in which two molecules are linked by dehydration condensation have higher energy than the sum of the energy of each single molecule. The phosphate bond of adenosine triphosphate (ATP), which is famous as an energy substance, has an energy of 7.3 kcal/mol. On the other hand, it seems to be about 0.5 kcal/mol in the case of peptide bonds, but it is higher than when it is disjointed. Energy does not naturally rise at amino acid linkage. It means that unless extra energy is brought in and injected from anywhere, it will never be connected. In addition, in order to actually carry out the reaction, “activation energy” which is even larger than the energy for bonding is required.
● Amino acids are chosen one by one and concatenated
Peptides are made using a large molecule tool called ribosome in the cytoplasm. Ribosomes ligate new amino acids in order to peptides that are made. However, amino acids that become a material do not swim by themselves. Amino acids are first attached at the C-terminus to the carrier molecule called transfer RNA (tRNA) specific for that amino acid. This chemical bond is linked by the enzyme, aminoacyl tRNA synthetase, using the energy of adenosine triphosphate (ATP) (*). The linkage between this amino acid and tRNA has higher energy than the activation energy of peptide bond between amino acids.
※ The reaction here is not ATP ⇒ ADP + Pi (phosphoric acid) which is common, but ATP ⇒ AMP + PPi (pyrophosphate).
The amino acid of the material enters the ribosome in a state connected with tRNA. When inside the ribosome, the C-terminus of the amino acid at the trailing end of the polypeptide to be made is bound to the tRNA that was previously attached. Once the N-terminus of the newly arrived amino acid is set near the C-terminus of the engineered polypeptide, the old tRNA is cut off from this C-terminus. Using the released energy, the N-terminus of the new amino acid is then ligated to the naked C-terminus. After this, it is the same repetition. By the function of ribosome and rRNA, new amino acids are added while being selected one by one for peptides, and a long chain polypeptide is made.
(※ This animation is completely omitting tRNA’s involvement.)
The resultant polypeptide is bent automatically by the influence of characteristics depending on the type and position of the constituent amino acid, takes a characteristic three-dimensional structure, and has a function peculiar to the polypeptide.
Will not the N terminus and the C terminus of the resultant polypeptide be connected to each other? I do not know whether chemical structure is possible, but for a cell that has only a mechanism to link amino acids one by one, its trick will be impossible. Another special mechanism is required to connect the trailing long polypeptide to the associated N terminus to the C terminus.
There is a fixed beginning of polypeptide
In the synthesis of the polypeptide, the N-terminal amino acid is the top. For any amino acid at the N terminal, it is possible to concatenate after any matter. However, at the stage of actually synthesizing the polypeptide, the starting amino acid is special. It is because the position is completely different between arriving at the back end of an already existing row and starting. After all, when synthesis starts, only the special tRNA that binds a specific amino acid called methionine can be headed to. Afterwards it is free to link various amino acids. Then, if all the resulting polypeptides are methionine at the beginning, that is not the case. Indeed, various amino acids are headed. At the end of the synthesis, the first methionine is truncated (if not necessary), and the second (and subsequent) amino acid is the beginning of the resulting polypeptide.
● Peptides are hydrolyzed to separate amino acids
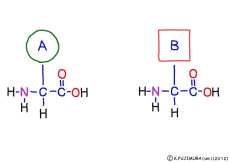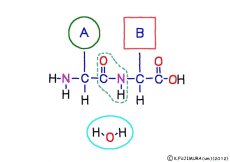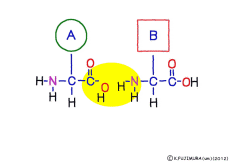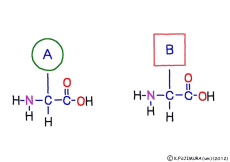
Peptide binding is one of dehydration condensation . 3 major nutrients carbohydrates , lipids, proteins are all It is made by dehydration condensation of element molecules. When they are ingested as foods and are digested and absorbed, they are dispersed into element molecules by hydrolysis, an opposite reaction, and are absorbed. Glycogen (starch) is decomposed into glucose, lipids into glycerin and fatty acids, and proteins into amino acids. It goes without saying that special enzymes and activation energy are necessary to proceed with this reaction. It hardly (almost) happens that it will degrade by itself when mixed in water.
● Peptide decomposition is unexpectedly troublesome
Even though the types of amino acids are different, the polypeptides are linked in the same peptide bond form, so if you think that decomposition will be easy in one way, in reality it is very troubling. To do decomposition work in the body, it is impossible without enzymatic protein to work, but enzymes are good at first, finding and deciding a certain structure of a certain size. The structure of each polypeptide differs greatly depending on its kind, and when it is cleaved somewhere, the structure of the cleaved peptide is changed, so that enzyme can not compete with it. There is no enzyme “cutting all peptide bonds” of one polypeptide. Therefore, several types of enzymes are involved in the degradation of polypeptides. Enzymes that roughly find large parts of the polypeptide, choke up, break down specific peptides, enzymes that clutter the chopped peptides, and finally the enzymes that separate amino acids one by onefrom the edge of a small peeled peptide.
○ Referenced sites
⇒20 kinds of amino acids in protein, room of living environment chemistry, 2017..
⇒Various functions (Ajinomoto) for each amino acid【Link broken】 ⇒ Ajinomoto · Amino Acid Encyclopedia, 2017 .:https://www.ajinomoto.co.jp/amino/.
⇒Ribosome:wikipedia.
⇒Transfer RNA:wikipedia
⇒Recently, about the topical collagen.
⇒Explanation of amino acids (MEXT)
○ Related articles
◆[007] Glucose and the hydrolysis of sucrose ![]()
◆[017] 糖質の吸収 absorption of carbohydratel ![]()
◆[019] アデノシン三リン酸(ATP) adenosine triphosphate ![]()
◆[011] Body acid-base reaction and carbon dioxide gas ![]()
◆[030] Endocytosis and intracellular digestion ![]()
◆[032] Glycogen synthesis ![]()
◆[046] Digestion and metabolism ![]()
○ Referenced books
・プロッパー細胞生物学,化学同人
・ワトソン遺伝子の分子生物学 第6版 東京電機大学出版局
・マッキー生化学―分子から解き明かす生命 化学同人
・Essential細胞生物学〈DVD付〉原書第3版,南江堂
・細胞の分子生物学, ニュートンプレス; 第5版 (2010/01)
・肉単―ギリシャ語・ラテン語 (語源から覚える解剖学英単語集 (筋肉編))
・カラー図解 人体の正常構造と機能 全10巻縮刷版,坂井 建雄,日本医事新報社
・人体機能生理学,杉 晴夫,南江堂
・トートラ人体解剖生理学 原書8版,丸善
・イラスト解剖学,松村 讓兒,中外医学社
・柔道整復学校協会編「生理学」,南江堂
・東洋療法学校協会編「生理学」,医歯薬出版株式会社
rev.20150909, rev.20160627, rev.20180130, rev.20180428, rev.20180429.
KISO-IGAKU-KYOIKU-KENKYUKAI(KIKKEN)